- anti-IL-25 monoclonal antibody candidate (LNR125.38) in development for treatment of CIP
- Unlike other CIP therapeutics, LNR125.38 significantly reduces tumor growth rather than promoting it
- Implications in all fibrotic and type 2 inflammatory conditions, with active R&D underway for pulmonary fibrosis, non-allergic asthma, atopic dermatitis, COPD, Ulcerative colitis, and others
- Licensing and partnership opportunities for additional indications
- Seasoned founding management has successfully launched multiple drugs
The Ideal Treatment for CIP
Protection from pulmonary fibrosis with tumor suppression
Executive Summary
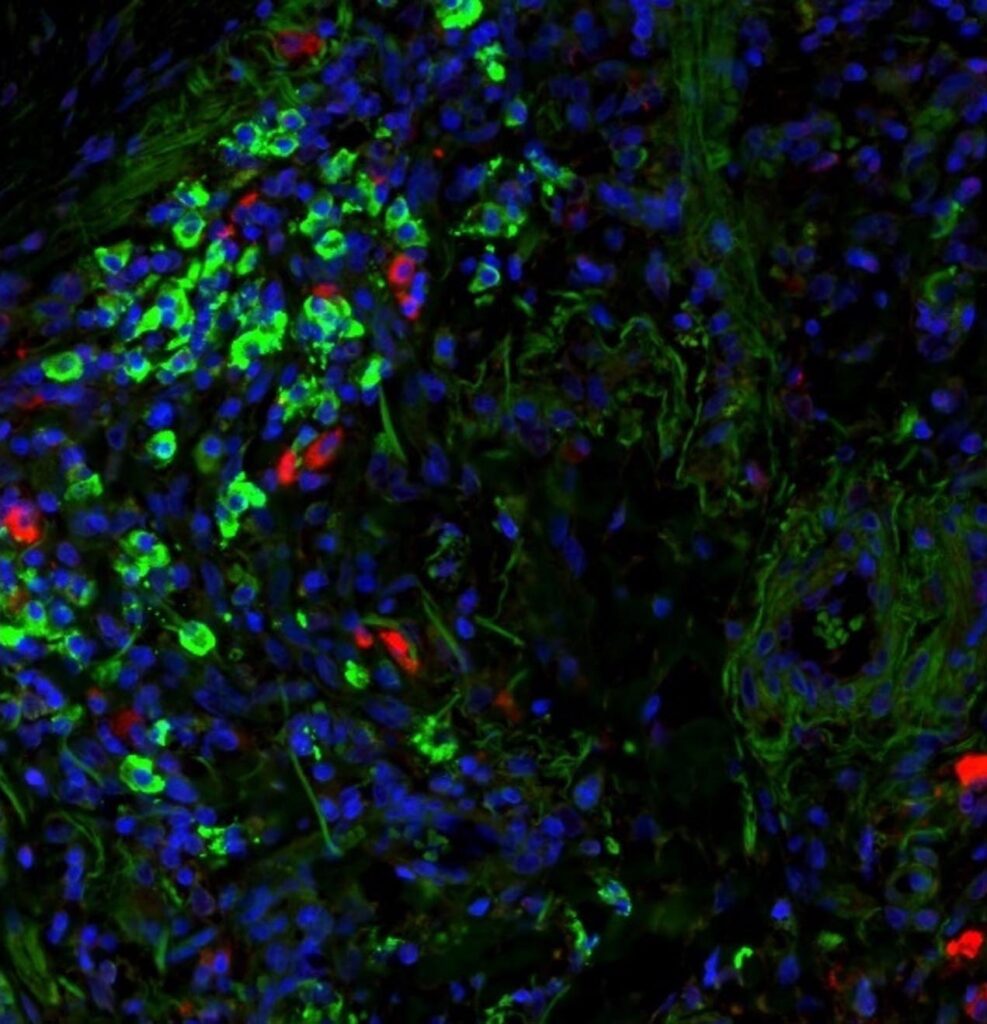
LEAD INDICATION
Checkpoint Inhibitor Induced Pneumonitis and Interstitial Lung Disease
Up to 30% of cancer patients taking immune checkpoint inhibitors (ICls) will develop pneumonitis as a result of this therapy as more combinations and chronic administration becomes the norm.
Over 20% of those with checkpoint inhibitor pneumonitis (CIP) will die from respiratory failure – with an average 1 month progression free survival for severe CIP.
LEAD INDICATION
Checkpoint Inhibitor Induced Pneumonitis and Interstitial Lung Disease
Up to 30% of cancer patients taking immune checkpoint inhibitors (ICls) will develop pneumonitis as a result of this therapy as more combinations and chronic administration becomes the norm.
Over 20% of those with checkpoint inhibitor pneumonitis (CIP) will die from respiratory failure – with an average 1 month progression free survival for severe CIP.
